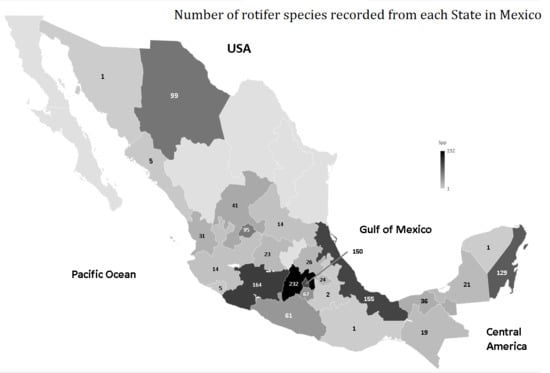
Rotifer Species Diversity in Mexico: An Updated Checklist
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Weber, D.; Hintermann, U.; Zangger, A. Scale and trends in species richness: Considerations for monitoring biological diversity for political purposes. Glob. Ecol. Biogeogr. 2004, 13, 97–104. [Google Scholar] [CrossRef]
- Llorente-Bousquets, J.; Ocegueda, S. Estado del conocimiento de la biota. In Capital Natural de México. Conocimiento Actual de la Biodiversidad; Conabio: México City, Mexico, 2008; Volume 1, pp. 283–322. [Google Scholar]
- Ramírez-Albores, J.E.; Badano, E.I.; Flores-Flores, J.; Yáñez-Espinosa, L. Scientific literature on invasive alien species in a megadiverse country: Advances and challenges in Mexico. NeoBiota 2019, 48, 113–127. [Google Scholar] [CrossRef]
- Morrone, J.J.; Escalante, T.; Rodríguez-Tapia, G. Mexican biogeographic provinces: Map and shapefiles. Zootaxa 2017, 4277, 277–279. [Google Scholar] [CrossRef]
- Wallace, R.L.; Snell, T.W.; Ricci, C.; Nogrady, T. Rotifera. In Biology, Ecology and Systematics, 2nd ed.; Segers, H., Ed.; Guides to the Identification of the Microinvertebrates of the Continental Waters of the World; Kenobi Productions: Ghent, Belgium; Backhuys Publishers: Leiden, The Netherlands, 2006; Volume 23. [Google Scholar]
- De la Lanza, E.G.; García, C.J.L. Lagos y Presas de México; AGT Editor S.A.: México City, Mexico, 2002; p. 680. [Google Scholar]
- Rico-Martínez, R.; Silva-Briano, M. Contribution to the knowledge of the rotifera of Mexico. Hydrobiologia 1993, 255/256, 467–474. [Google Scholar] [CrossRef]
- Rico-Martínez, R. Rotíferos. In La Biodiversidad en Aguascalientes: Estudio de Estado; Comisión Nacional para el Conocimiento y Uso de la Biodiversidad (CONABIO): Aguascalientes, Mexico; Instituto del Medio Ambiente del Estado de Aguascalientes (IMAE): Aguascalientes, Mexico; Universidad Autónoma de Aguascalientes (UAA): Aguascalientes, Mexico, 2008. [Google Scholar]
- Sarma, S.S.S.; Serranía-Soto, C.; Nandini, S. Diversidad de Rotíferos. In La Diversidad Biológica del Estado de México; Ceballos, G.R., Ed.; Gobierno del Estado de México y Comisión Nacional para el Conocimiento y Uso de la Biodiversidad (CONABIO): México, Mexico, 2009. [Google Scholar]
- Castellanos-Páez, M.E.; Zamora-García, M.; Benítez-Díaz-Mirón, M.I.; Mouriño, G.G.; Contreras-Tapia, R.A. Abundancia y biomasa de la comunidad de rotíferos y su relación con parámetros ambientales en tres estaciones del Canal Cuemanco, Xochimilco. Soc. Rural. Prod. Medio Ambiente 2014, 14, 10–21. [Google Scholar]
- Arroyo-Castro, J.L.; Alvarado-Flores, J.; Uh-Moo, J.C.; Koh-Pasos, C.G. Monogonont rotifers species of the island Cozumel, Quintana Roo, México. Biodivers. Data J. 2019, 7, e34719. [Google Scholar] [CrossRef]
- Ortega-Murillo, M.d.R.; Alvarado-Villanueva, R.; Sánchez-Heredia, J.D.; Muñóz-Gaytán, A.A.; Morales, R.H. El Plancton de Agua dulce. In La Biodiversidad en Michoacán; Estudio de Estado 2. CONABIO; Comisión Nacional Para el Conocimiento y Uso de la Biodiversidad (CONABIO): México, Mexico, 2019. [Google Scholar]
- Delgado-Saucedo, J.J.; Silva-Briano, M.; Sigala-Rodríguez, J.J. Rotíferos. In La Biodiversidad en Zacatecas; Estudio de Estado. CONABIO; Comisión Nacional para el Conocimiento y Uso de la Biodiversidad (CONABIO): México, Mexico; Gobierno del Estado de Zacatecas: México, Mexico, 2020. [Google Scholar]
- Sarma, S.S.S. Checklist of rotifers (Rotifera) from Mexico. Environ. Ecol. 1999, 17, 978–983. [Google Scholar]
- Gwinn, D.C.; Allen, M.S.; Bonvechio, K.I.; Hoyer, M.V.; Beesley, L.S. Evaluating estimators of species richness: The importance of considering statistical error rates. Methods Ecol. Evol. 2016, 7, 294–302. [Google Scholar] [CrossRef]
- Segers, H. Annotated checklist of the rotifers (Phylum Rotifera), with notes on nomenclature, taxonomy and distribution. Zootaxa 2007, 1564, 1–104. [Google Scholar] [CrossRef]
- Jersabek, C.D.; Leitner, M.F. The Rotifer World Catalog. World Wide Web Electronic Publication. 2013. Available online: http://www.rotifera.hausdernatur.at/ (accessed on 20 May 2021).
- Alcántara-Rodríguez, J.A.; Ciros-Pérez, J.; Ortega-Mayagoitia, E.; Serrania-Soto, C.R.; Piedra-Ibarra, E. Local adaptation in populations of a Brachionus group plicatilis cryptic species inhabiting three deep crater lakes in Central Mexico. Freshw. Biol. 2012, 57, 728–740. [Google Scholar] [CrossRef]
- Brown, P.D.; Schröder, T.; Ríos-Arana, J.V.; Rico-Martínez, R.; Silva-Briano, M.; Wallace, R.L.; Walsh, E.J. Patterns of rotifer diversity in the Chihuahuan desert. Diversity 2020, 12, 393. [Google Scholar] [CrossRef]
- Koste, W. Rotatoria. In Die Rädertiere Mitteleuropas. Ein Bestimmungswerk Begründet von Max Voigt; Bornträger: Stuttgart, Germany, 1978; Volume 1–2, pp. 234, 673. [Google Scholar]
- Segers, H. Rotifera 2: The Lecanidae Monogononta; Guides to the Identification of Microinvertebrates of the Continental Waters of the World Series; Balogh Scientific Books: The Hague, The Netherlands, 1994; Volume 6. [Google Scholar]
- Wallace, R.L.; Snell, T.W. Rotifera. Chapter 8. In Ecology and Classifications of North American Freshwater Invertebrates, 3rd ed.; Thorp, J., Covich, A., Eds.; Elsevier: Oxford, UK, 2010. [Google Scholar]
- Wallace, R.L.; Snell, T.W.; Walsh, E.J.; Sarma, S.S.S.; Segers, H. Chapter 8. Phylum Rotifera. Keys to Palaearctic Fauna. In Thorp and Covich’s Freshwater Invertebrates, 4th ed.; Academic Press: Cambridge, MA, USA; Elsevier: Amsterdam, The Netherlands, 2019; Volume 4, pp. 219–267. [Google Scholar] [CrossRef]
- Colwell, R.K.; Mao, C.X.; Chang, J. Interpolating, extrapolating, and comparing incidence-based species accumulation curves. Ecology 2004, 85, 2717–2727. [Google Scholar] [CrossRef] [Green Version]
- García-Morales, A.E.; Elías-Gutiérrez, M. DNA barcoding of freshwater rotifera in Mexico: Evidence of cryptic speciation in common rotifers. Mol. Ecol. Resour. 2013, 13, 1097–1107. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Contreras, J.; Sarma, S.S.S.; Piedra-Ibarra, E.; Calderón-Torres, M.; Nandini, S. Morphological, morphometrical and molecular (CO1 and ITS) analysis of the rotifer Asplanchna brightwellii from selected freshwater bodies in Central Mexico (Mexico). J. Environ. Biol. 2013, 34, 1039–1046. [Google Scholar]
- Jiménez-Contreras, J.; Sarma, S.S.S.; Piedra-Ibarra, E.; Nandini, S. Morphometric and molecular (COX 1) variations of Asplanchna girodi clones from Central Mexico. J. Environ. Biol. 2017, 38, 1229–1239. [Google Scholar] [CrossRef]
- Nandini, S.; Peña-Aguado, F.; Arreguin-Rebolledo, U.; Sarma, S.S.S.; Murugan, G. Molecular identity and demographic responses to salinity of a freshwater strain of Brachionus plicatilis from the shallow Lake Pátzcuaro, Mexico. Fundam. Appl. Limnol. 2019, 192, 319–329. [Google Scholar] [CrossRef]
- Kordbacheh, A.; Shapiro, A.N.; Walsh, E.J. Reproductive isolation, morphological and ecological differentiation among cryptic species of Euchlanis dilatata, with the description of four new species. Hydrobiologia 2019, 844, 221–242. [Google Scholar] [CrossRef]
- Walsh, E.J.; Schröder, T.; Wallace, R.L.; Rico-Martínez, R. Cryptic speciation in Lecane bulla (Monogononta: Rotifera) in Chihuahuan Desert waters. Int. Ver. Für Theor. Angew. Limnol. Verh. 2009, 30, 1046–1050. [Google Scholar]
- Nandini, S.; Merino-Ibarra, M.; Sarma, S.S.S. Seasonal changes in the zooplankton abundances of the reservoir Valle de Bravo (State of Mexico, Mexico). Lake Reserv. Manag. 2008, 24, 321–330. [Google Scholar] [CrossRef] [Green Version]
- Moreno-Gutiérrez, R.M.; Sarma, S.S.S.; Sobrino-Figueroa, A.S.; Nandini, S. Population growth potential of rotifers from a high altitude eutrophic waterbody, Madín reservoir (State of Mexico, Mexico): The importance of seasonal sampling. J. Limnol. 2018, 77, 441–451. [Google Scholar] [CrossRef]
- Muñoz-Colmenares, M.E.; Sarma, S.S.S.; Nandini, S. Seasonal variation of rotifers from the high altitude Llano reservoir (State of Mexico, Mexico). J. Environ. Biol. 2017, 38, 1171–1181. [Google Scholar] [CrossRef]
- Sarma, S.S.S.; Osnaya-Espinosa, L.R.; Aguilar-Acosta, C.R.; Nandini, S. Seasonal variations in zooplankton abundances in the Iturbide reservoir (Isidro Fabela, State of Mexico, Mexico). J. Environ. Biol. 2011, 32, 473–480. [Google Scholar]
- Figueroa-Sanchez, M.A.; Nandini, S.; Sarma, S.S.S. Zooplankton community structure in the presence of low levels of cyanotoxins: A case study in a high altitude tropical reservoir (Valley de Bravo, Mexico). J. Limnol. 2014, 73, 157–166. [Google Scholar] [CrossRef] [Green Version]
- García-García, G.; Nandini, S.; Sarma, S.S.S.; Martínez-Jerónimo, F.; Jiménez-Contreras, J. Impact of chromium and aluminium pollution on the diversity of zooplankton: A case study in the Chimaliapan wetland (Ramsar site) (Lerma basin, Mexico). J. Environ. Sci. Health Part. A 2012, 47, 534–547. [Google Scholar] [CrossRef]
- Jiménez-Contreras, J.; Nandini, S.; Sarma, S.S.S. Diversity of Rotifera (Monogononta) and egg ratio of selected taxa in the canals of Xochimilco (Mexico City). Wetlands 2018, 38, 1033–1044. [Google Scholar] [CrossRef]
- Gutiérrez, S.G.; Sarma, S.S.S.; Nandini, S. Seasonal variations of rotifers from a high altitude urban shallow water body, La Cantera Oriente (Mexico City, Mexico). Chin. J. Oceanol. Limnol. 2017, 35, 1387–1397. [Google Scholar] [CrossRef]
- Espinosa-Rodríguez, C.A.; Sarma, S.S.S.; Nandini, S. Zooplankton community changes in relation to different macrophyte species: Effects of Egeria densa removal. Ecohydrol. Hydrobiol. 2021, 21, 153–163. [Google Scholar] [CrossRef]
- Nandini, S.; Sarma, S.S.S.; Gulati, R.D. A seasonal study reveals the occurrence of exotic rotifers in the river Antigua, Veracruz, close to the Gulf of Mexico. River Res. Appl. 2017, 33, 970–982. [Google Scholar] [CrossRef]
- Nandini, S.; Ramírez-García, P.; Sarma, S.S.S.; Gutiérrez-Ochoa, R.A. Planktonic indicators of water quality: A case study in the Amacuzac River Basin (State of Morelos, Mexico). River Res. Appl. 2019, 35, 268–279. [Google Scholar] [CrossRef]
- Vázquez-Sánchez, A.; Reyes-Vanegas, G.; Nandini, S.; Sarma, S.S.S. Diversity and abundance of rotifers (Rotifera) during an annual cycle in the reservoir Valerio Trujano (Tepecoacuilco, Mexico). Inland Waters 2004, 4, 293–302. [Google Scholar] [CrossRef]
- Suárez-Morales, E.; Vázquez-Mazy, A.; Solís, M.E. Preliminary investigations on the zooplankton community of a Mexican eutrophic reservoir, a seasonal survey. Hidrobiológica 1993, 3, 71–80. [Google Scholar]
- Ramírez-García, P.; Nandini, S.; Sarma, S.S.S.; Robles-Valderrama, E.; Cuesta, I.; Hurtado-Maria, D. Seasonal variations of zooplankton abundance in the freshwater reservoir Valle de Bravo (Mexico). Hydrobiologia 2002, 467, 99–108. [Google Scholar] [CrossRef]
- Mills, S.J.; Alcántara-Rodríguez, A.; Ciros-Pérez, J.; Gómez, A.; Hagiwara, A.; Galindo, K.H.; Jersabek, C.D.; Malekzadeh-Viayeh, R.; Leasi, F.; Lee, J.-S.; et al. Fifteen species in one: Deciphering the Brachionus plicatilis species complex (Rotifera, Monogononta) through DNA taxonomy. Hydrobiologia 2017, 796, 39–58. [Google Scholar] [CrossRef] [Green Version]
- Elías-Gutiérrez, M.; Suárez-Morales, E.; Sarma, S.S.S. Diversity of freshwater zooplankton in the neotropics: The case of Mexico. Verh. Internat. Verein. Limnol. 2001, 27, 4027–4031. [Google Scholar] [CrossRef]
- Ríos-Arana, J.V.; del Carmen Agüero-Reyes, L.; Wallace, R.L.; Walsh, E.J. Limnological characteristics and rotifer community composition of Northern Mexico Chihuahuan desert springs. J. Arid. Environ. 2019, 160, 32–41. [Google Scholar] [CrossRef]
- Walsh, E.J.; Schröder, T.; Wallace, R.L.; Ríos-Arana, J.V.; Rico-Martínez, R. Rotifers from selected inland saline waters in the Chihuahuan Desert of México. Saline Syst. 2008, 4, 7. [Google Scholar] [CrossRef] [Green Version]
- Sarma, S.S.S.; Nandini, S.; Ramírez-García, P.; Cortés-Muñoz, J.E. New records of brackish water Rotifera and Cladocera from Mexico. Hidrobiológica 2000, 10, 121–124. [Google Scholar]
- Escobar-Briones, E.; Villalobos-Hiriart, J.L. Nebalia lagartensis (Leptostraca) a new species from the Yucatán Peninsula, Mexico. Crustaceana 1995, 68, 1–11. [Google Scholar]
- Nandini, S.; Sarma, S.S.S. Adaptive toe morphology of Euchlanis cf. mikropous Koch-Althaus, 1962 (Rotifera: Euchlanidae) exposed directly and indirectly to invertebrate predators. Limnologica 2019, 78, 125693. [Google Scholar] [CrossRef]
- Sarma, S.S.S.; Jiménez-Santos, M.A.; Nandini, S.; Wallace, R.L. Review on the ecology and taxonomy of sessile rotifers (Rotifera) with special reference to Mexico. J. Environ. Biol. 2020, 41, 3–12. [Google Scholar] [CrossRef]
- Gabaldón, C.; Fontaneto, D.; Carmona, M.J.; Montero-Pau, J.; Serra, M. Ecological differentiation in cryptic rotifer species: What we can learn from the Brachionus plicatilis complex. Hydrobiologia 2017, 796, 7–18. [Google Scholar] [CrossRef]
- Kordbacheh, A.; Wallace, R.L.; Walsh, E.J. Evidence supporting cryptic species within two sessile microinvertebrates, Limnias melicerta and L ceratophylli (Rotifera, Gnesiotrocha). PLoS ONE 2018, 13, e0205203. [Google Scholar] [CrossRef]


| Subclass: Bdelloidea Hudson, 1884 |
| Order: Adinetida Melone & Ricci, 1995 |
| Family: Adinetidae Hudson & Gosse, 1886 |
| 1 Adineta vaga (Davis, 1873) |
| Order: Philodinida Melone & Ricci 1995 |
| Family: Philodinidae Ehrenberg, 1838 |
| 2 Dissotrocha aculeata (Ehrenberg, 1830) |
| 3 Macrotrachela sonorensis Örstan, 1995 |
| 4 Philodina acuticornis Murray, 1902 |
| 5 Philodina megalotrocha Ehrenberg, 1832 |
| 6 Philodina roseola Ehrenberg, 1832 |
| Philodina cf. roseola Ehrenberg, 1832 |
| 7 Pleuretra africana Murray, 1911 |
| 8 Rotaria citrina (Ehrenberg, 1838) |
| 9 Rotaria elongata (Weber, 1888) |
| 10 Rotaria magnacalcarata (Parsons, 1892) |
| 11 Rotaria neptunia (Ehrenberg, 1830) |
| 12 Rotaria rotatoria (Pallas, 1766) |
| Subclass: Monogononta Plate, 1889 |
| Order: Collothecacea Harring, 1913 |
| Family: Atrochidae Harring, 1913 |
| 13 Atrochus tentaculatus Wierzejski, 1893 |
| 14 Cupelopagis vorax (Leidy, 1857) |
| Family: Collothecidae Harring, 1913 |
| 15 Collotheca ambigua (Hudson, 1883) |
| 16 Collotheca campanulata (Dobie, 1849) |
| 17 Collotheca coronetta (Cubitt, 1869) |
| 18 Collotheca crateriformis Offord, 1934 |
| 19 Collotheca ornata (Ehrenberg, 1832) |
| 20 Collotheca pelagica (Rousselet, 1893) |
| 21 Collotheca riverai Vilaclara & Sládeček, 1989 |
| 22 Collotheca tenuilobata (Anderson, 1889) |
| 23 Collotheca trilobata (Collins, 1872) |
| 24 Stephanoceros millsii (Kellicott, 1885) |
| Order: Flosculariacea Harring, 1913 |
| Family: Conochilidae Harring, 1913 |
| 25 Conochilus coenobasis (Skorikov, 1914) |
| 26 Conochilus dossuarius Hudson, 1885 |
| 27 Conochilus hippocrepis (Schrank, 1803) |
| 28 Conochilus natans (Seligo, 1900) |
| 29 Conochilus unicornis Rousselet, 1892 |
| Family: Flosculariidae Ehrenberg, 1838 |
| 30 Beauchampia crucigere (Dutrochet, 1812) |
| 31 Floscularia melicerta (Ehrenberg, 1832) |
| 32 Limnias ceratophylli Schrank, 1803 |
| 33 Limnias melicerta Weisse, 1848 |
| 34 Octotrocha speciosa Thorpe, 1893 |
| 35 Ptygura beauchampi Edmondson, 1940 |
| 36 Ptygura brachiata (Hudson, 1886) |
| 37 Ptygura brevis (Rousselet, 1893) |
| 38 Ptygura crystallina (Ehrenberg, 1834) |
| 39 Ptygura furcillata (Kellicott, 1889) |
| 40 Ptygura libera Myers, 1934 |
| 41 Ptygura cf. linguata Edmondson, 1939 |
| 42 Ptygura longicornis (Davis, 1867) |
| 43 Ptygura melicerta Ehrenberg, 1832 |
| 44 Ptygura pedunculata Edmondson, 1939 |
| 45 Ptygura tacita Edmondson, 1940 |
| 46 Ptygura tridorsicornis Summerfiel-Wright, 1957 |
| 47 Ptygura velata (Gosse, 1851) |
| 48 Sinantherina ariprepes Edmondson, 1939 |
| 49 Sinantherina semibullata (Thorpe, 1893) |
| 50 Sinantherina socialis (Linnaeus, 1758) |
| Family: Hexarthridae Bartoš, 1959 |
| 51 Hexarthra fennica (Levander, 1892) |
| 52 Hexarthra intermedia (Wiszniewski, 1929) |
| Hexarthra intermedia f. braziliensis Hauer, 1953 |
| 53 Hexarthra jenkinae (de Beauchamp, 1932) |
| 54 Hexarthra mira (Hudson, 1871) |
| 55 Hexarthra oxyuris (Sernov, 1903) |
| 56 Hexarthra polyodonta (Hauer, 1957) |
| Family: Testudinellidae Harring, 1913 |
| 57 Pompholyx complanata Gosse, 1851 |
| 58 Pompholyx sulcata Hudson, 1885 |
| 59 Testudinella caeca (Parsons, 1892) |
| 60 Testudinella emarginula (Stenroos, 1898) |
| 61 Testudinella incisa (Ternetz, 1892) |
| 62 Testudinella mucronata (Gosse, 1886) |
| 63 Testudinella parva (Ternetz, 1892) |
| 64 Testudinella patina (Hermann, 1783) |
| 65 Testudinella reflexa (Gosse, 1887) |
| Family: Trochosphaeridae Harring, 1913 |
| 66 Filinia brachiata (Rousselet, 1901) |
| 67 Filinia cornuta (Weisse, 1847) |
| 68 Filinia longiseta (Ehrenberg, 1834) |
| 69 Filinia opoliensis (Zacharias, 1898) |
| 70 Filinia pejleri Hutchinson, 1964 |
| 71 Filinia saltator (Gosse, 1886) |
| 72 Filinia terminalis (Plate, 1886) |
| 73 Horaella thomassoni Koste, 1973 |
| 74 Trochosphaera aequatorialis Semper, 1872 |
| Order: Ploima Hudson & Gosse, 1886 |
| Family: Asplanchnidae Eckstein, 1883 |
| 75 Asplanchna brightwellii Gosse, 1850 |
| 76 Asplanchna girodi de Guerne, 1888 |
| 77 Asplanchna herrickii de Guerne, 1888 |
| 78 Asplanchna intermedia Hudson, 1886 |
| 79 Asplanchna priodonta Gosse, 1850 |
| 80 Asplanchna sieboldii (Leydig, 1854) |
| 81 Asplanchna silvestrii Daday, 1902 |
| 82 Asplanchnopus multiceps (Schrank, 1793) |
| Family: Brachionidae Ehrenberg, 1838 |
| 83 Anuraeopsis fissa Gosse, 1851 |
| 84 Anuraeopsis quadriantennata (Koste, 1974) |
| 85 Brachionus ahlstromi Lindeman, 1939 |
| 86 Brachionus angularis Gosse, 1851 |
| 87 Brachionus araceliae Silva-Briano, Galván-De la Rosa, Pérez-Legaspi & Rico-Martínez, 2007 |
| 88 Brachionus bidentatus Anderson, 1889 |
| 89 Brachionus budapestinensis Daday, 1885 |
| 90 Brachionus calyciflorus Pallas, 1766 |
| Brachionus calyciflorus calyciflorus Pallas, 1766 |
| 91 Brachionus caudatus Barrois & Daday, 1894 |
| 92 Brachionus dimidiatus Bryce, 1931 |
| 93 Brachionus dolabratus Harring, 1914 |
| 94 Brachionus durgae Dhanapathi, 1974 |
| 95 Brachionus falcatus Zacharias, 1898 |
| 96 Brachionus forficula Wierzejski, 1891 |
| 97 Brachionus havanaensis Rousselet, 1911 |
| 98 Brachionus josefinae Silva-Briano & Segers, 1992 |
| 99 Brachionus leydigii Cohn, 1862 |
| 100 Brachionus paranguensis Guerrero-Jiménez, Vannucchi, Silva-Briano, Adabache-Ortiz, Rico-Martínez, Roberts, Neilson & Elías-Gutiérrez, 2019 |
| 101 Brachionus plicatilis Müller, 1786 |
| Brachionus plicatilis longicornis Fadeev, 1925 |
| 102 Brachionus pterodinoides Rousselet, 1913 |
| 103 Brachionus quadridentatus Hermann, 1783 |
| Brachionus quadridentatus quadridentatus Herman, 1783 |
| 104 Brachionus rotundiformis Tschugunoff, 1921 |
| 105 Brachionus rubens Ehrenberg, 1838 |
| 106 Brachionus urceolaris Müller, 1773 |
| 107 Brachionus variabilis Hempel, 1896 |
| 108 Kellicottia bostoniensis (Rousselet, 1908) |
| 109 Kellicottia longispina (Kellicott, 1879) |
| 110 Keratella americana Carlin, 1943 |
| 111 Keratella cochlearis (Gosse, 1851) |
| Keratella cochlearis cochlearis (Gosse, 1851) |
| 112 Keratella hiemalis Carlin, 1943 |
| 113 Keratella irregularis (Lauterborn, 1898) |
| 114 Keratella lenzi Hauer, 1953 |
| 115 Keratella mexicana Kutikova & Silva-Briano, 1995 |
| 116 Keratella morenoi Modenutti, Diéguez & Segers, 1998 |
| 117 Keratella procurva (Thorpe, 1891) |
| Keratella procurva robusta Koste & Shiel, 1980 |
| 118 Keratella quadrata (Müller, 1786) |
| 119 Keratella serrulata (Ehrenberg, 1838) |
| 120 Keratella taurocephala Myers, 1938 |
| 121 Keratella tecta (Gosse, 1851) |
| 122 Keratella ticinensis (Callerio, 1921) |
| 123 Keratella tropica (Apstein, 1907) |
| 124 Keratella valga (Ehrenberg, 1834) |
| 125 Notholca acuminata (Ehrenberg, 1832) |
| 126 Notholca bipalium (Müller, 1786) |
| 127 Notholca foliacea (Ehrenberg, 1838) |
| 128 Notholca cf. liepetterseni Godske Björklund, 1972 |
| 129 Notholca squamula (Müller, 1786) |
| 130 Notholca striata (Müller, 1786) |
| 131 Plationus patulus (Daday, 1905) |
| Plationus patulus macracanthus (Müller, 1786) |
| 132 Plationus polyacanthus (Ehrenberg, 1834) |
| 133 Platyias leloupi Gillard, 1967 |
| 134 Platyias quadricornis (Ehrenberg, 1832) |
| Family: Dicranophoridae Harring, 1913 |
| 135 Aspelta angusta Harring & Myers, 1928 |
| 136 Aspelta curvidactyla Bērziņš, 1949 |
| 137 Aspelta lestes Harring & Myers, 1928 |
| 138 Dicranophoroides caudatus (Ehrenberg, 1834) |
| 139 Dicranophoroides claviger (Hauer, 1965) |
| 140 Dicranophorus epicharis Harring & Myers, 1928 |
| 141 Dicranophorus forcipatus (Müller, 1786) |
| 142 Dicranophorus grandis (Ehrenberg, 1832) |
| 143 Dicranophorus prionacis Harring & Myers, 1928 |
| 144 Dicranophorus robustus Harring & Myers, 1928 |
| 145 Encentrum cf. cruentum Harring & Myers, 1928 |
| 146 Encentrum saundersiae (Hudson, 1885) |
| 147 Encentrum uncinatum (Milne, 1886) |
| 148 Paradicranophorus sordidus Donner, 1968 |
| Family: Epiphanidae Harring, 1913 |
| 149 Cyrtonia tuba (Ehrenberg, 1834) |
| 150 Epiphanes brachionus (Ehrenberg, 1837) |
| 151 Epiphanes clavulata (Ehrenberg, 1832) |
| 152 Epiphanes macroura (Barrois & Daday, 1894) |
| 153 Epiphanes senta (Müller, 1773) |
| 154 Proalides subtilis Rodewald, 1940 |
| 155 Proalides tentaculatus de Beauchamp, 1907 |
| Family: Euchlanidae Ehrenberg, 1838 |
| 156 Beauchampiella eudactylota (Gosse, 1886) |
| 157 Dipleuchlanis elegans (Wierzejski, 1893) |
| 158 Dipleuchlanis propatula (Gosse, 1886) |
| 159 Euchlanis calpidia Myers, 1930 |
| 160 Euchlanis deflexa (Gosse, 1851) |
| 161 Euchlanis dilatata Ehrenberg, 1832 |
| Euchlanis dilatata lucksiana Hauer, 1930 |
| 162 Euchlanis incisa Carlin, 1939 |
| 163 Euchlanis lyra Hudson, 1886 |
| 164 Euchlanis cf. mikropous Koch-Althaus, 1962 |
| 165 Euchlanis oropha Gosse, 1887 |
| 166 Euchlanis pyriformis Gosse, 1851 |
| 167 Euchlanis triquetra Ehrenberg, 1838 |
| 168 Tripleuchlanis plicata (Levander, 1894) |
| Family: Gastropodidae Harring, 1913 |
| 169 Ascomorpha ecaudis Perty, 1850 |
| 170 Ascomorpha ovalis (Bergendal, 1892) |
| 171 Ascomorpha saltans Bartsch, 1870 |
| 172 Gastropus hyptopus (Ehrenberg, 1838) |
| 173 Gastropus stylifer (Imhof, 1891) |
| Family: Ituridae Sudzuki, 1964 |
| 174 Itura aurita (Ehrenberg, 1830) |
| 175 Itura chamadis Harring & Myers, 1928 |
| 176 Itura myersi Wulfert, 1935 |
| Family: Lecanidae Remane, 1933 |
| 177 Lecane aculeata (Jakubski, 1912) |
| 178 Lecane aeganea Harring, 1914 |
| 179 Lecane arcuata (Bryce, 1891) |
| 180 Lecane arcula Harring, 1914 |
| 181 Lecane aspasia Myers, 1917 |
| 182 Lecane bifurca (Bryce, 1892) |
| 183 Lecane bulla (Gosse, 1851) |
| 184 Lecane candida Harring & Myers, 1926 |
| 185 Lecane clara (Bryce, 1892) |
| 186 Lecane closterocerca (Schmarda, 1859) |
| 187 Lecane cornuta (Müller, 1786) |
| 188 Lecane crenata (Harring, 1913) |
| 189 Lecane crepida Harring, 1914 |
| 190 Lecane curvicornis (Murray, 1913) |
| 191 Lecane decipiens (Murray, 1913) |
| 192 Lecane doryssa Harring, 1914 |
| 193 Lecane elasma Harring & Myers, 1926 |
| 194 Lecane elegans Harring, 1914 |
| 195 Lecane elsa Hauer, 1931 |
| 196 Lecane flexilis (Gosse, 1886) |
| 197 Lecane furcata (Murray, 1913) |
| 198 Lecane grandis (Murray, 1913) |
| 199 Lecane haliclysta Harring & Myers, 1926 |
| 200 Lecane hamata (Stokes, 1896) |
| 201 Lecane hastata (Murray, 1913) |
| Lecane cf. hastata (Murray, 1913) |
| 202 Lecane hornemanni (Ehrenberg, 1834) |
| 203 Lecane inermis (Bryce, 1892) |
| 204 Lecane inopinata Harring & Myers, 1926 |
| 205 Lecane latissima Yamamoto, 1955 |
| 206 Lecane leontina (Turner, 1892) |
| 207 Lecane levistyla (Olofsson, 1917) |
| 208 Lecane ludwigii (Eckstein, 1883) |
| 209 Lecane luna (Müller, 1776) |
| 210 Lecane lunaris (Ehrenberg, 1832) |
| 211 Lecane margarethae Segers, 1991 |
| 212 Lecane monostyla (Daday, 1897) |
| 213 Lecane nana (Murray, 1913) |
| 214 Lecane nelsoni Segers, 1994 |
| 215 Lecane obtusa (Murray, 1913) |
| 216 Lecane ohioensis (Herrick, 1885) |
| 217 Lecane papuana (Murray, 1913) |
| 218 Lecane perpusilla (Hauer, 1929) |
| 219 Lecane pertica Harring & Myers, 1926 |
| 220 Lecane punctata (Murray, 1913) |
| 221 Lecane pyriformis (Daday, 1905) |
| 222 Lecane quadridentata (Ehrenberg, 1830) |
| 223 Lecane rhenana Hauer, 1929 |
| 224 Lecane rhytida Harring & Myers, 1926 |
| 225 Lecane rugosa (Harring, 1914) |
| 226 Lecane ruttneri Hauer, 1938 |
| 227 Lecane satyrus Harring & Myers, 1926 |
| 228 Lecane scutata (Harring & Myers, 1926) |
| 229 Lecane signifera (Jennings, 1896) |
| 230 Lecane sola Hauer, 1936 |
| 231 Lecane spinulifera Edmondson, 1935 |
| 232 Lecane stenroosi (Meissner, 1908) |
| 233 Lecane stichaea Harring, 1913 |
| 234 Lecane stokesii (Pell, 1890) |
| 235 Lecane subtilis Harring & Myers, 1926 |
| 236 Lecane subulata (Harring & Myers, 1926) |
| 237 Lecane tenuiseta Harring, 1914 |
| 238 Lecane thalera (Harring & Myers, 1926) |
| 239 Lecane thienemanni (Hauer, 1938) |
| 240 Lecane uenoi Yamamoto, 1951 |
| 241 Lecane undulata Hauer, 1938 |
| 242 Lecane unguitata (Fadeev, 1925) |
| 243 Lecane ungulata (Gosse, 1887) |
| 244 Lecane venusta Harring & Myers, 1926 |
| 245 Lecane yatseni Wei & Xu, 2010 |
| Family: Lepadellidae Harring, 1913 |
| 246 Colurella adriatica Ehrenberg, 1831 |
| 247 Colurella colurus (Ehrenberg, 1830) |
| 248 Colurella hindenburgi Steinecke, 1917 |
| 249 Colurella oblonga Donner, 1943 |
| 250 Colurella obtusa (Gosse, 1886) |
| 251 Colurella uncinata (Müller, 1773) |
| Colurella uncinata bicuspidata (Ehrenberg, 1832) |
| 252 Lepadella acuminata (Ehrenberg, 1834) |
| 253 Lepadella apsida Harring, 1916 |
| 254 Lepadella astacicola Hauer, 1926 |
| 255 Lepadella benjamini Harring, 1916 |
| 256 Lepadella biloba Hauer, 1958 |
| 257 Lepadella cf. cornuta Koste & Shiel, 1989 |
| 258 Lepadella cristata (Rousselet, 1893) |
| 259 Lepadella dactyliseta (Stenroos, 1898) |
| 260 Lepadella discoidea Segers, 1993 |
| 261 Lepadella donneri Koste, 1972 |
| 262 Lepadella ehrenbergii (Perty, 1850) |
| 263 Lepadella heterostyla (Murray, 1913) |
| 264 Lepadella latusinus (Hilgendorf, 1899) |
| 265 Lepadella ovalis (Müller, 1786) |
| 266 Lepadella patella (Müller, 1773) |
| Lepadella patella patella (Müller, 1786) |
| 267 Lepadella punctata Wulfert, 1939 |
| 268 Lepadella quadricarinata (Stenroos, 1898) |
| 269 Lepadella quinquecostata (Lucks, 1912) |
| Lepadella quinquecostata quinquecostata (Lucks, 1912) |
| 270 Lepadella rhomboides (Gosse, 1886) |
| 271 Lepadella triba Myers, 1934 |
| 272 Lepadella triptera (Ehrenberg, 1832) |
| 273 Squatinella lamellaris (Müller, 1786) |
| Family: Lindiidae Harring & Myers, 1924 |
| 274 Lindia ecela Myers, 1933 |
| 275 Lindia tecusa Harring & Myers, 1922 |
| 276 Lindia torulosa Dujardin, 1841 |
| 277 Lindia truncata (Jennings, 1894) |
| Family: Mytilinidae Harring, 1913 |
| 278 Lophocharis oxysternon (Gosse, 1851) |
| 279 Lophocharis salpina (Ehrenberg, 1834) |
| 280 Mytilina acanthophora Hauer, 1938 |
| 281 Mytilina bisulcata (Lucks, 1912) |
| 282 Mytilina mucronata (Müller, 1773) |
| Mytilina mucronata spinigera (Ehrenberg, 1830) |
| 283 Mytilina ventralis (Ehrenberg, 1830) |
| Mytilina ventralis brevispina (Ehrenberg, 1830) |
| Mytilina ventralis ventralis (Ehrenberg, 1830) |
| Family: Notommatidae Hudson & Gosse, 1886 |
| 284 Cephalodella apocolea Myers, 1924 |
| 285 Cephalodella calosa Wulfert, 1956 |
| 286 Cephalodella catellina Müller, 1786 |
| 287 Cephalodella exigua (Gosse, 1886) |
| 288 Cephalodella forficula (Ehrenberg, 1830) |
| 289 Cephalodella gibba (Ehrenberg, 1830) |
| 290 Cephalodella gigantea Remane, 1933 |
| 291 Cephalodella globata (Gosse, 1887) |
| 292 Cephalodella gracilis (Ehrenberg, 1830) |
| 293 Cephalodella cf. graciosa Wulfert, 1956 |
| 294 Cephalodella hoodii (Gosse, 1886) |
| 295 Cephalodella macrodactyla (Stenroos, 1898) |
| 296 Cephalodella cf. marina Myers, 1924 |
| 297 Cephalodella megalocephala (Glascott, 1893) |
| 298 Cephalodella misgurnus Wulfert, 1937 |
| 299 Cephalodella panarista Myers, 1924 |
| 300 Cephalodella physalis Myers, 1924 |
| Cephalodella cf. physalis Myers, 1924 |
| 301 Cephalodella rotunda Wulfert, 1937 |
| 302 Cephalodella stenroosi Wulfert, 1937 |
| 303 Cephalodella sterea (Gosse, 1887) |
| 304 Cephalodella tenuiseta (Burn, 1890) |
| 305 Cephalodella ventripes (Dixon-Nuttall, 1901) |
| 306 Enteroplea lacustris Ehrenberg, 1830 |
| 307 Eosphora anthadis Harring & Myers, 1922 |
| 308 Eosphora ehrenbergi Weber & Montet, 1918 |
| 309 Eosphora najas Ehrenberg, 1830 |
| 310 Eosphora thoa Harring & Myers, 1830 |
| 311 Eosphora thoides Wulfert, 1935 |
| 312 Eothinia carogaensis Myers, 1937 |
| 313 Eothinia elongata (Ehrenberg, 1832) |
| 314 Monommata actices Remane, 1933 |
| 315 Monommata diaphora Myers, 1930 |
| 316 Notommata aurita (Müller, 1786) |
| 317 Notommata cerberus (Gosse, 1886) |
| 318 Notommata copeus Ehrenberg, 1834 |
| 319 Notommata cyrtopus Gosse, 1886 |
| 320 Notommata falcinella Harring & Myers, 1922 |
| 321 Notommata glyphura Wulfert, 1935 |
| 322 Notommata haueri Wulfert, 1939 |
| Notommata cf. haueri Wulfert, 1939 |
| 323 Notommata pachyura (Gosse, 1886) |
| 324 Notommata saccigera Ehrenberg, 1830 |
| 325 Notommata tripus Ehrenberg, 1838 |
| 326 Pleurotrocha petromyzon (Ehrenberg, 1830) |
| 327 Resticula gelida (Harring & Myers, 1922) |
| 328 Resticula melandocus (Gosse, 1887) |
| 329 Resticula nyssa Harring & Myers, 1924 |
| 330 Sphyrias lofauna (Rousselet, 1910) |
| 331 Taphrocampa annulosa Gosse, 1851 |
| 332 Taphrocampa selenura Gosse, 1887 |
| Family: Proalidae Harring & Myers, 1924 |
| 333 Proales cognita Myers, 1940 |
| 334 Proales daphnicola Thompson, 1892 |
| 335 Proales decipiens (Ehrenberg, 1832) |
| 336 Proales fallaciosa Wulfert, 1937 |
| 337 Proales globulifera (Hauer, 1921) |
| 338 Proales sigmoidea (Skorikov, 1896) |
| 339 Proales similis de Beauchamp, 1907 |
| 340 Proales sordida Gosse, 1886 |
| 341 Proales cf. wesenbergi Wulfert, 1960 |
| 342 Wulfertia ornata Donner, 1943 |
| Family: Scaridiidae Manfredi, 1927 |
| 343 Scaridium botsjani Daems & Dumont, 1974 |
| 344 Scaridium longicaudum (Müller, 1786) |
| Family: Synchaetidae Hudson & Gosse, 1886 |
| 345 Ploesoma hudsoni (Imhof, 1891) |
| 346 Polyarthra dolichoptera Idelson, 1925 |
| Polyarthra cf. dolichoptera Idelson, 1925 |
| 347 Polyarthra euryptera Wierzejski, 1891 |
| 348 Polyarthra longiremis Carlin, 1943 |
| 349 Polyarthra luminosa Kutikova, 1962 |
| 350 Polyarthra major Burckhardt, 1900 |
| 351 Polyarthra remata Skorikov, 1896 |
| 352 Polyarthra trigla Ehrenberg, 1834 (species inquirenda) |
| 353 Polyarthra vulgaris Carlin, 1943 |
| 354 Synchaeta bicornis Smith, 1904 |
| 355 Synchaeta elsteri Hauer, 1963 |
| 356 Synchaeta hyperborea Smirnov, 1932 |
| 357 Synchaeta longipes Gosse, 1887 |
| 358 Synchaeta oblonga Ehrenberg, 1832 |
| 359 Synchaeta pectinata Ehrenberg, 1832 |
| 360 Synchaeta stylata Wierzejski, 1893 |
| 361 Synchaeta tremula (Müller, 1786) |
| 362 Synchaeta tremuloida Pourriot, 1965 |
| Family: Tetrasiphonidae Harring & Myers, 1924 |
| 363 Tetrasiphon hydrocora Ehrenberg, 1840 |
| Family: Trichocercidae Harring, 1913 |
| 364 Ascomorphella volvocicola (Plate, 1886) |
| 365 Trichocerca bicristata (Gosse, 1887) |
| 366 Trichocerca bidens (Lucks, 1912) |
| 367 Trichocerca brachyura (Gosse, 1851) |
| 368 Trichocerca braziliensis (Murray, 1913) |
| 369 Trichocerca capucina (Wierzejski & Zacharias, 1893) |
| 370 Trichocerca collaris (Rousselet, 1896) |
| 371 Trichocerca cylindrica (Imhof, 1891) |
| 372 Trichocerca dixonnuttalli (Jennings, 1903) |
| 373 Trichocerca elongata (Gosse, 1886) |
| 374 Trichocerca iernis (Gosse, 1887) |
| 375 Trichocerca insignis (Herrick, 1885) |
| 376 Trichocerca insulana (Hauer, 1937) |
| 377 Trichocerca cf. intermedia (Stenroos, 1898) |
| 378 Trichocerca longiseta (Schrank, 1802) |
| 379 Trichocerca marina (Daday, 1890) |
| 380 Trichocerca mollis Edmondson, 1936 |
| 381 Trichocerca mucosa (Stokes, 1896) |
| 382 Trichocerca multicrinis (Kellicott, 1897) |
| 383 Trichocerca musculus (Hauer, 1937) |
| 384 Trichocerca porcellus (Gosse, 1851) |
| 385 Trichocerca pusilla (Jennings, 1903) |
| 386 Trichocerca rattus (Müller, 1776) |
| 387 Trichocerca rosea (Stenroos, 1898) |
| 388 Trichocerca rousseleti (Voigt, 1902) |
| 389 Trichocerca ruttneri Donner, 1953 |
| 390 Trichocerca similis (Wierzejski, 1893) |
| 391 Trichocerca stylata (Gosse, 1851) |
| 392 Trichocerca tenuior (Gosse, 1886) |
| 393 Trichocerca tigris (Müller, 1786) |
| 394 Trichocerca vernalis (Hauer, 1936) |
| 395 Trichocerca weberi (Jennings, 1903) |
| Family: Trichotriidae Harring, 1913 |
| 396 Macrochaetus collinsii (Gosse, 1867) |
| 397 Macrochaetus longipes Myers, 1934 |
| 398 Macrochaetus sericus (Thorpe, 1893) |
| 399 Macrochaetus subquadratus (Perty, 1850) |
| 400 Trichotria pocillum (Müller, 1776) |
| 401 Trichotria tetractis (Ehrenberg, 1830) |
| 402 Wolga spinifera (Western, 1894) |
| Species Complex | Reference |
|---|---|
| Ascomorpha ovalis | [25] |
| Asplanchna brightwellii | [26] |
| Asplanchna girodi | [27] |
| Brachionus calyciflorus | [25] |
| Brachionus plicatilis | [18,28] |
| Brachionus quadridentatus | [25] |
| Euchlanis dilatata | [29] |
| Keratella cochlearis | [25] |
| Lecane bulla | [30] |
| Lecane cornuta | [25] |
| Lecane crepida | [25] |
| Lecane curvicornis | [25] |
| Lecane hastata | [25] |
| Lecane lunaris | [25] |
| Mytilina ventralis | [25] |
| Platyias quadricornis | [25] |
| Testudinella patina | [25] |
| Species/States | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 | 25 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adineta | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Anuraeopsis | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 2 | 1 | 0 | 1 | 0 | 1 |
| Ascomorpha | 3 | 0 | 0 | 1 | 0 | 2 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 2 | 0 | 1 | 0 | 3 | 1 | 0 | 3 | 0 | 0 |
| Ascomorphella | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 |
| Aspelta | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 1 | 0 | 0 |
| Asplanchna | 5 | 0 | 1 | 0 | 0 | 2 | 2 | 0 | 1 | 4 | 3 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 6 | 2 | 0 | 4 | 0 | 1 |
| Asplanchnopus | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Atrochus | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Beauchampia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Beauchampiella | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 |
| Brachionus | 14 | 4 | 5 | 5 | 0 | 4 | 11 | 6 | 2 | 13 | 14 | 8 | 0 | 0 | 0 | 8 | 2 | 0 | 0 | 11 | 9 | 1 | 17 | 0 | 12 |
| Cephalodella | 2 | 0 | 0 | 12 | 0 | 0 | 2 | 0 | 0 | 4 | 8 | 3 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 14 | 0 | 0 | 9 | 0 | 0 |
| Collotheca | 1 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 5 | 2 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 |
| Colurella | 0 | 0 | 0 | 4 | 0 | 0 | 0 | 0 | 0 | 3 | 2 | 2 | 1 | 0 | 0 | 3 | 0 | 0 | 0 | 4 | 0 | 0 | 5 | 0 | 0 |
| Conochilus | 4 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 0 | 0 | 2 | 0 | 1 |
| Cupelopagis | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Cyrtonia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Dicranophoroides | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 2 | 0 | 0 |
| Dicranophorus | 0 | 0 | 0 | 2 | 0 | 0 | 1 | 0 | 0 | 2 | 1 | 2 | 1 | 0 | 0 | 3 | 0 | 0 | 0 | 4 | 0 | 1 | 3 | 0 | 0 |
| Dipleuchlanis | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 2 | 0 | 0 | 1 | 0 | 0 |
| Encentrum | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 |
| Enteroplea | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 |
| Eosphora | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 3 | 1 | 0 | 2 | 0 | 0 |
| Eothinia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Epiphanes | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 2 | 2 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 2 | 1 | 0 | 2 | 0 | 0 |
| Euchlanis | 2 | 0 | 0 | 3 | 0 | 1 | 1 | 2 | 0 | 5 | 2 | 1 | 0 | 0 | 0 | 2 | 1 | 0 | 0 | 6 | 1 | 2 | 4 | 0 | 0 |
| Filinia | 4 | 0 | 2 | 3 | 0 | 2 | 3 | 0 | 1 | 4 | 3 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 5 | 1 | 1 | 2 | 0 | 3 |
| Floscularia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Gastropus | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 |
| Hexarthra | 2 | 0 | 1 | 2 | 0 | 2 | 1 | 0 | 1 | 2 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 3 | 0 | 0 | 2 | 0 | 0 |
| Horaëlla | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 |
| Itura | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 3 | 0 | 0 | 1 | 0 | 0 |
| Kellicottia | 2 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 1 |
| Keratella | 10 | 0 | 3 | 3 | 0 | 4 | 3 | 5 | 2 | 5 | 11 | 3 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 10 | 3 | 4 | 5 | 1 | 5 |
| Lecane | 10 | 13 | 2 | 21 | 3 | 0 | 15 | 3 | 0 | 29 | 29 | 21 | 17 | 0 | 0 | 40 | 6 | 1 | 0 | 34 | 4 | 2 | 39 | 0 | 11 |
| Lepadella | 2 | 0 | 0 | 5 | 1 | 1 | 1 | 4 | 0 | 6 | 11 | 5 | 1 | 0 | 0 | 9 | 0 | 2 | 0 | 11 | 0 | 2 | 5 | 0 | 1 |
| Limnias | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Lindia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 2 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 2 | 0 | 0 | 2 | 0 | 0 |
| Lophocharis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 2 | 0 | 0 | 1 | 0 | 0 |
| Macrochaetus | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 |
| Monommata | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Mytilina | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 3 | 3 | 1 | 1 | 0 | 0 | 3 | 0 | 0 | 0 | 4 | 0 | 0 | 4 | 0 | 0 |
| Notholca | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 2 | 0 | 0 |
| Notommata | 0 | 0 | 0 | 2 | 0 | 0 | 1 | 0 | 0 | 1 | 4 | 0 | 3 | 0 | 0 | 3 | 0 | 0 | 0 | 7 | 0 | 0 | 2 | 0 | 0 |
| Octotrocha | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Paradicranophorus | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Philodina | 1 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Plationus | 2 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 2 | 0 | 0 | 1 | 0 | 1 |
| Platyias | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 2 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 2 | 1 | 0 | 1 | 0 | 1 |
| Pleuretra | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Pleurotrocha | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 0 |
| Ploesoma | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Polyarthra | 5 | 0 | 1 | 2 | 0 | 2 | 2 | 1 | 3 | 3 | 4 | 1 | 0 | 0 | 0 | 1 | 2 | 0 | 0 | 6 | 0 | 1 | 1 | 0 | 0 |
| Pompholyx | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 |
| Proales | 1 | 0 | 0 | 5 | 0 | 0 | 0 | 0 | 0 | 2 | 1 | 1 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 3 | 0 | 0 | 1 | 0 | 0 |
| Proalides | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Ptygura | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 8 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 0 |
| Resticula | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 |
| Rotaria | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 1 | 0 | 0 |
| Scaridium | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 |
| Sinantherina | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 2 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 2 | 0 | 1 | 1 | 0 | 0 |
| Sphyrias | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Squatinella | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 |
| Stephanoceros | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Synchaeta | 4 | 0 | 1 | 2 | 0 | 0 | 0 | 0 | 1 | 3 | 4 | 1 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 3 | 4 | 1 | 1 | 0 | 0 |
| Taphrocampa | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 2 | 0 | 1 | 1 | 0 | 0 |
| Testudinella | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 3 | 5 | 2 | 1 | 0 | 0 | 4 | 0 | 0 | 0 | 6 | 0 | 1 | 3 | 0 | 0 |
| Tetrasiphon | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Trichocerca | 7 | 0 | 0 | 2 | 0 | 1 | 3 | 0 | 0 | 15 | 12 | 4 | 2 | 0 | 0 | 8 | 0 | 1 | 0 | 23 | 6 | 2 | 10 | 0 | 1 |
| Trichotria | 2 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 2 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 1 | 2 | 0 | 2 |
| Tripleuchlanis | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 |
| Trochosphaera | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| Wolga | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Wulfertia | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Waterbody | Total Species | Reference |
|---|---|---|
| Valle de Bravo Reservoir (State of Mexico) | 50 | [31] |
| Madín reservoir (State of Mexico) | 28 | [32] |
| Llano reservoir (State of Mexico) | 84 | [33] |
| Iturbide reservoir (State of Mexico) | 55 | [34] |
| Lake Zumpango (State of Mexico) | 33 | [35] |
| Chimaliapan wetland (State of Mexico) | 75 | [36] |
| Lake Xochimilco (Mexico City) | 81 | [37] |
| Lake Cantera Oriente (Mexico City) | 68 | [38] |
| Benito Juárez Reservoir (Mexico City) | 80 | [39] |
| River Antigua (Veracruz State) | 125 | [40] |
| Amacuzac River Basin (State of Morelos) | 65 | [41] |
| Valerio Trujano Reservoir (Guerrero State) | 64 | [42] |
| Species and Distribution | Records from Mexico |
|---|---|
| Adineta vaga: Afr, Pal | Quintana Roo and Tlaxcala: Neo |
| Atrochus tentaculatus: Aus, Pal, Ori | Mexico City: Nea |
| Collotheca crateriformis: Pal | Chihuahua: Nea |
| Colurella colurus: Pal | State of Mexico and Chihuahua: Nea; Veracruz and Quintana Roo: Neo |
| Colurella oblonga: Pal | Veracruz: Neo |
| Dicranophorus forcipatus: Pal | State of Mexico, Michoacán, Mexico City, Chihuahua and Tlaxcala: Nea; Morelos, Veracruz, Quintana Roo, Guerrero and Nayarit: Neo |
| Epiphanes brachionus: Pal | Mexico City: Nea; Guerrero: Neo |
| Horaella thomassoni: Neo | State of Mexico, Michoacán and Aguascalientes: Nea |
| Keratella procurva robusta: Aus | State of Mexico, Michoacán and Aguascalientes: Nea and Tabasco: Neo |
| Lecane unguitata: Afr, Aus, Ori, Pal | State of Mexico, Michoacán and Mexico City: Nea, Quintana Roo and Veracruz: Neo |
| Lecane yatseni: Ori | Veracruz: Neo |
| Lepadella discoidea: Afr, Aus, Ori | State of Mexico: Nea |
| Lepadella punctata: Ori, Pal | State of Mexico: Nea |
| Mytilina mucronata spinigera: Pal | Aguascalientes: Nea |
| Mytilina ventralis: Afr, Pac, Pal | State of Mexico, Mexico City, Morelos, Michoacán: Nea; Veracruz, Quintana Roo and Nayarit: Neo |
| Notholca acuminata: Afr, Pal | Chihuahua: Nea |
| Notommata haueri: Pal | Chihuahua: Nea |
| Paradicranophorus sordidus: Ant, Pal | Chihuahua: Nea |
| Philodina acuticornis: Pal | Chihuahua: Nea |
| Plationus polyacanthus: Pal | State of Mexico and Aguascalientes: Nea |
| Proales globulifera: Pal | State of Mexico: Nea |
| Ptygura brevis: Aus, Pal | Chihuahua deserts: Nea |
| Ptygura tridorsicornis: Pal | State of Mexico: Nea |
| Sphyrias lofauna: Afr, Pac | Michoacán: Nea |
| Squatinella lamellaris: Pac, Pal | State of Mexico, Michoacán and Mexico City: Nea; Veracruz y Quintana Roo: Neo |
| Synchaeta elsteri: Pal | Michoacán: Nea |
| Synchaeta hyperborea: Pal | Tabasco: Neo |
| Synchaeta tremuloida: Pal | Jalisco: Nea |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarma, S.S.S.; Jiménez-Santos, M.A.; Nandini, S. Rotifer Species Diversity in Mexico: An Updated Checklist. Diversity 2021, 13, 291. https://doi.org/10.3390/d13070291
Sarma SSS, Jiménez-Santos MA, Nandini S. Rotifer Species Diversity in Mexico: An Updated Checklist. Diversity. 2021; 13(7):291. https://doi.org/10.3390/d13070291
Chicago/Turabian StyleSarma, S. S. S., Marco Antonio Jiménez-Santos, and S. Nandini. 2021. "Rotifer Species Diversity in Mexico: An Updated Checklist" Diversity 13, no. 7: 291. https://doi.org/10.3390/d13070291